Ellagitannins with glucopyranose cores have higher affinities to proteins than acyclic ellagitannins by isothermal titration calorimetry
Recently, we published a paper looking at the ellagitannin-protein interactions and their thermodynamics by isothermal titration calorimetry (ITC):
ITC can measure the thermodynamics of ellagitannin-protein interactions
ITC is a sophisticated and fancy technique to measure the biological binding interactions as it can be used to measure the thermodynamics of the interaction, i.e. the binding constant, the enthalpy of binding, and the stoichiometry or number of binding sites. In this study, 12 individual ellagitannins and two different proteins, namely BSA and gelatin, were used and the structural features affecting the interaction between the ellagitannin and the protein revealed.
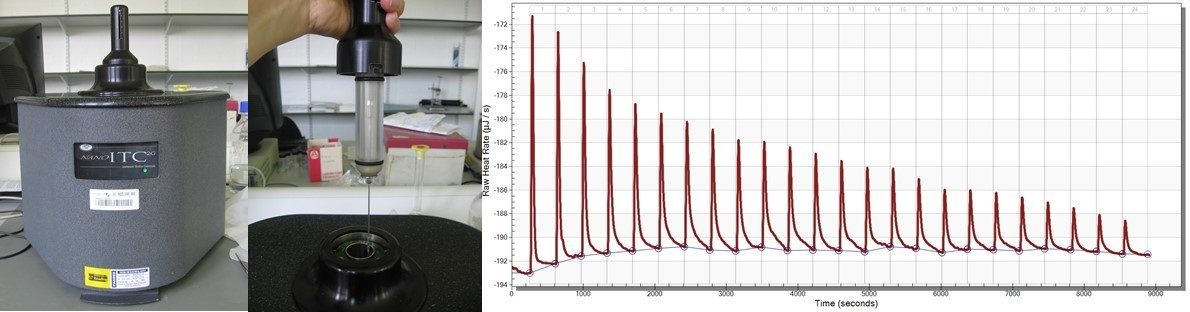
In the isothermal titration calorimeter (A), the ellagitannin solution is in the injection syringe in the burette (B) and titrated into the protein solution in the sample cell. The end result is a thermogram (C), that is, the plot of heat against the injection number.
Ellagitannins have mainly two-site binding with gelatin, whereas with BSA the secondary interaction are very weak
The interaction of ellagitannins were stronger with gelatin than with BSA. In general, ellagitannins with glucopyranose cores had higher affinity to both proteins than acyclic ellagitannins. For the interactions with gelatin, the primary stronger binding sites and the secondary weaker binding sites were detected. Whereas, for the interaction with BSA, the secondary interactions were found to be very weak. In addition, free galloyls, the molecular flexibility and higher molecular size of ellagitannins enhanced the strength of the interaction. Smaller structural features did not show any trend.
Acknowledgements
The research project was multidisciplinary and it was carried out in collaboration with professor Rebecca J. Green and professor Irene Mueller-Harvey from University of Reading, UK. The research was funded by the Academy of Finland (grants 251388 and 310549 to Maarit Karonen).
