The partition coefficients between n-octanol and water of 47 hydrolysable tannins were measured utilizing UPLC and HPLC with UV detection.
Recently, we studied the hydrophobicity of hydrolysable tannins with a large set of hydrolysable tannins in order to reveal and understand the structural features affecting their hydrophobicity that would help in estimating the biological activity of these compounds better. The results provide predictions as to which types of HT structures are more hydrophobic and thus more likely to interact efficiently for example with lipid membranes:
Hydrophobicity of hydrolysable tannins determined by partition coefficient measurements
The studied group of hydrolysable tannins consisted of 6 galloyl glucoses, 4 gallotannins, 9 simple HHDP-esters, 10 C-glycosidic ellagitannins, 2 dehydroellagitannins, 3 modified dehydroellagitannins and 13 ellagitannin oligomers. The analysis of partition coefficient measurements was done with both HPLC and UPLC
with three replicates and the octanol-water partition coefficients (logP) were determined as the decadic logarithm of the ratio of the concentration of analyte in the octanol phase to the concentration of analyte in the water phase (Figure 1). The measured logP values (see Table A1 in the original publication) were used in the structure-activity pattern evaluations.
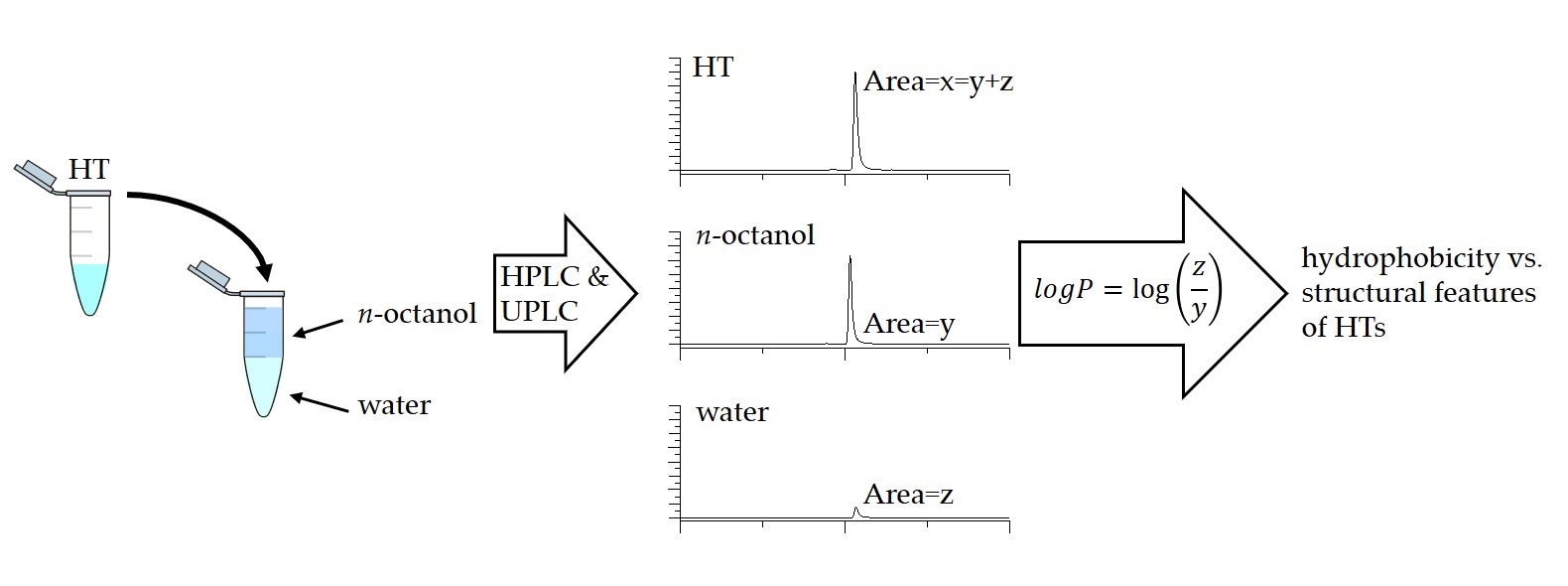
Figure 1. The partition coefficients were measured with the shake flask method utilizing both HPLC and UPLC.
Structural features affecting the hydrophobicity of hydrolysable tannins
Results showed that galloyl glucoses and gallotannins were clearly more hydrophobic than ellagitannins but the differences in hydrophobicity within ellagitannins were more varied than within galloyl glucoses or gallotannins (Figure 2). Most notable structural features that were found to influence the hydrophobicity of ellagitannins were the number of free galloyl groups, acyclic versus cyclic polyol, substitution of the anomeric position of glucose and 4C1 versus 1C4 conformation of the glucopyranose core.
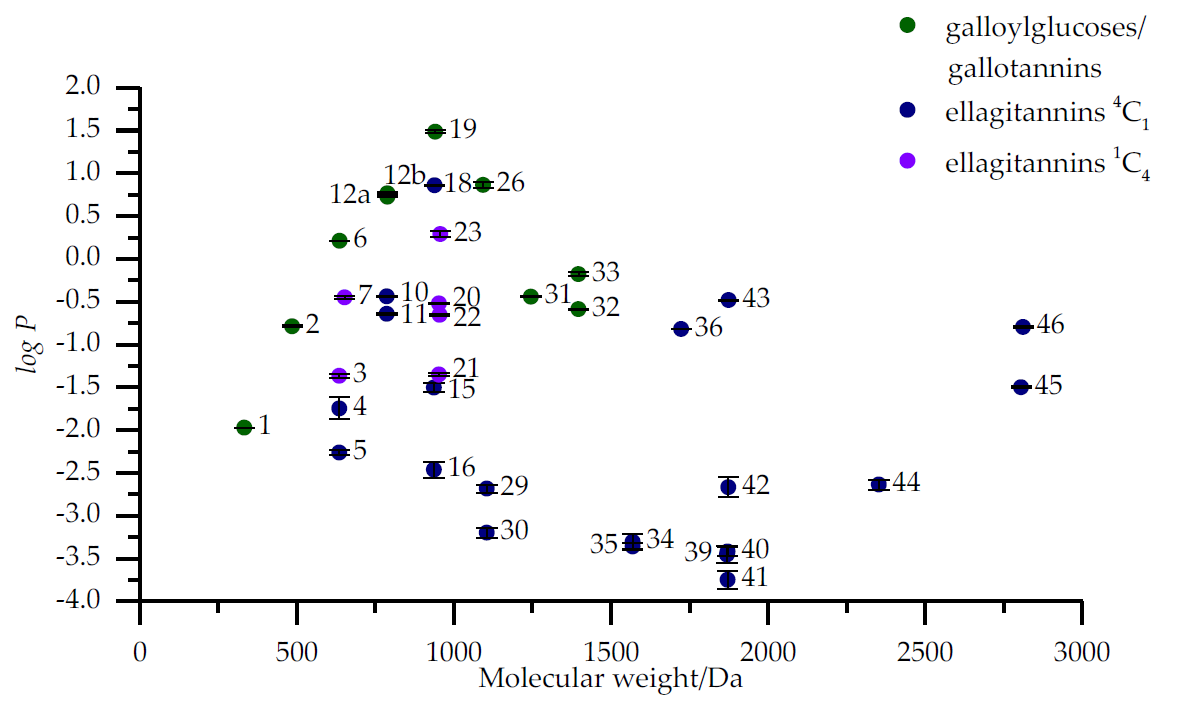
Figure 2. LogP values of 47 hydrolysable tannins plotted against their molecular weights measured with UPLC showing galloylglucoses/gallotannins, 4C1 glucose core ellagitannins and 1C4 glucose core ellagitannins in different series. The numbers of individual hydrolysable tannins refer to the Figures 1 and 2 and Table A1 in the original publication.
In general, as the number of free galloyl groups in the structures of hydrolysable tannins increased, also their hydrophobicity increased. Hydrolysable tannins with glucopyranose cores had mainly higher hydrophobicities than C-glycosidic ones possibly due to the very rigid geometries many of these C-glycosidic ETs. Within the glucopyranose-based ellagitannins, the ones having the energetically unfavorable 1C4 configuration generally had higher hydrophobicities than the ellagitannins with 4C1 glucopyranose cores, but this feature was not as significant as the number of free galloyl groups. The substitution of the anomeric position of the polyol glucose also proved to be an important factor in both the glucopyranose based and C-glycosidic ellagitannins. The galloyl group in the anomeric position rather than in any other position increased the hydrophobicity of glucopyranose based hydrolysable tannins comparatively more and, in the C-glycosidic ellagitannins, the α anomer proved to be more hydrophobic than the corresponding β anomer.
Acknowledgements
This research was funded by the Academy of Finland, grant number 310549 to Maarit Karonen.
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
mons.org/licenses/by/4.0/).
