Structural features of hydrolysable tannins reveal their capacity to precipitate a model protein
Our long term efforts at the Natural Chemistry Research Group regarding structure-activity studies of hydrolysable tannins (HTs) have resulted in a new scientific paper by Engström et al. (2019) reporting the ability of 32 purified and characterized HTs to form insoluble complexes with model protein, bovine serum albumin. This research was a true team effort with major input by our great MSc students Joona Arvola, Saara Nenonen and Valtteri Virtanen. The whole paper can be found at:
A new turbidimetric 96-well plate reader method was developed for studies of tannin-protein interactions
Tannin bioactivities cannot be studied without accurate analysis tools. We created a rapid in-house well-plate reader method to study how the formation of insoluble complexes between proteins and tannins are dependent on the specific structural details of hydrolysable tannins and proanthocyanidins, another sub-group of tannins. The method is also suitable for the measurement of the protein precipitation capacities of crude plant extracts. It can be thus used for both compound-specific studies as well as for large screening experiments with hundreds or even thousands of plant samples.
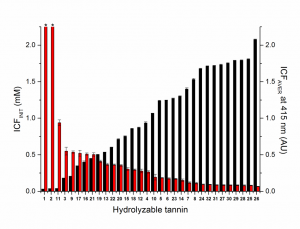
The average insoluble complex formation of the whole concentration range (ICFAVER, black columns) and the initiation concentration for the insoluble complex formation i.e. the x-intercept (ICFINIT, red columns) for the studied hydrolysable tannins.
32 hydrolysable tannins were purified from the main biosynthetic branches
It is unusual to have a number of purified HTs in tannin-protein studies. For the time-consuming purification step, we chose altogether 32 compounds from multiple branches of the HT biosynthesis pathway so that we could make conclusions how this bioactivity of HTs changes while their biosynthesis progresses. At the same time this enabled us to make broader conclusions on the bioactivities of plant species utilizing these specific HT pathways.
Clear structure-activity patterns were found and an equation created for activity predictions
The results showed clear relationship between the HT structure and the intensity of haze that formed during the tannin–protein complexation. In addition to molecular weight, structural features such as number of galloyl groups, degree of oxidative coupling between the galloyls, positional isomerism and cyclic vs acyclic glucose core, affected the ability of the monomeric HTs to form insoluble complexes with bovine serum albumin. While oligomers were superior to monomers in their capability to precipitate the model protein, their activity depended less on the functional groups, but mostly on their size and overall flexibility.
The results allowed to construct an equation that predicted the protein precipitation capacity of the studied HTs with high accuracy. This was a great addition to our previous equations that were able to predict oxidative activities and anthelmintic activities of these same types of tannins.
Biosynthetic pathway matters for the tannin activity
We have previously shown that the oxidative activity of HTs changes logically along their proposed biosynthetic pathway. Engström et al. (2019) showed this to be true also for the protein binding activity of HTs. Regarding the distribution of the protein precipitation capacity along the biosynthetic pathway of HTs, the progression of the galloylglucose and gallotannin biosynthesis as well as all steps leading to oligomeric HTs yielded HTs with higher protein precipitation capacities. On the contrary, each oxidative step along the suggested biosynthetic pathway of ETs reduced the protein precipitation capacity as did the opening of the cyclic glucose core to yield C-glycosidic ETs. Most interestingly, an opposite trend has been observed for the oxidative activities of monomeric HTs. Altogether, in a bigger picture, the current results further supported our previous studies showing that the protein precipitation capacities and oxidative activities of monomeric HTs are negatively correlated; HTs efficient in forming insoluble complexes with proteins have lower oxidative activities and vice versa.
There is more to come
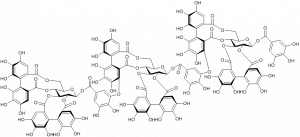
The most efficient hydrolyzable tannin to form complexes with the model protein, bovine serum albumin, was the trimeric ellagitannin lambertianin C.
We now know how the structure of the HTs affects their protein precipitation capacities and what are the molar ratios required for initiation of haze formation between different types of HTs and BSA. However, this study did not yet yield information on the stability of the formed insoluble complexes nor the effect of pH in the complex formation. These aspects will be the obvious next steps to investigate to better understand the role of tannins in, e.g., protecting dietary proteins from ruminal degradation and further liberation of proteins in the intestine of ruminants as pH and the nature of tannin–protein interactions change. Altogether, our current results form a good platform for choosing a more limited array of HTs into more detailed mechanistic studies of tannin–protein complexation.
