UV spectra of polyphenols are very important and practically the primary information that is obtained for an unknown plant component
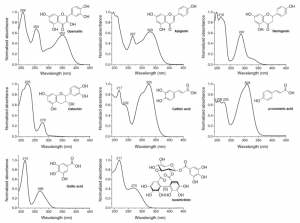 Different polyphenol subclasses have quite distinct chromophores in their structures, although naturally some common features can be found as well. Still, UV spectra obtained e.g. by LC-DAD are especially useful in the first characterization step that aims to classify the detected compound to belong in one of the polyphenol sub-groups. In most cases this is “easily” possible and more detailed look at the UV spectrum may reveal information of the possible structural features within the sub-group. This is especially true with ellagitannins, although it has been said that their UV spectra are “non-informative”. This is much due to the fact that UV spectra of polyphenols are rarely published and this hinders the wider understanding of the structural causes for their UV spectral shapes.
Different polyphenol subclasses have quite distinct chromophores in their structures, although naturally some common features can be found as well. Still, UV spectra obtained e.g. by LC-DAD are especially useful in the first characterization step that aims to classify the detected compound to belong in one of the polyphenol sub-groups. In most cases this is “easily” possible and more detailed look at the UV spectrum may reveal information of the possible structural features within the sub-group. This is especially true with ellagitannins, although it has been said that their UV spectra are “non-informative”. This is much due to the fact that UV spectra of polyphenols are rarely published and this hinders the wider understanding of the structural causes for their UV spectral shapes.
Of course the quality of the UV spectra is also dependent on the solution in which the spectra are recorded and on the concentration of the analytes that are being measured. With LC-DAD-MS instruments the biggest problem in this respect is related to formic acid that is one of the most commonly used eluents in polyphenol analysis. The problem is especially pronounced if aqueous formic acid concentrations are exceeded above 0.1%. In these cases very dilute LC peaks do not give very useful UV spectra as formic acid distorts the spectra at 190-230 nm region. As the peak intensity rises, the effect of formic acid gradually diminishes. This way only the peaks that are intensive enough, are able to produce such UV spectra that we use e.g. in the examples below. However, also the effect of formic acid can get less annoying as the user gets more familiar with the UV spectra of polyphenols in general.
