In contrast to the rather limited distribution of gallotannins in nature, ellagitannins are typical constituents of many plant families, but only two steps of their biosynthesis are characterized by enzyme studies
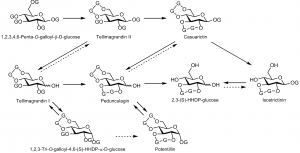
The first biosynthetic steps in the formation of ellagitannins from 1,2,3,4,6-penta-O-galloyl-beta-D-glucose onwards. The letter G is used to describe a galloyl unit, and G-G to describe an HHDP unit.
To date, over 500 structurally characterised ETs have been identified. This structural diversity is a consequence of the almost inexhaustible number of combinations and permutations involving intra-(C-C) and inter-(C-O) molecular oxidative couplings available to the ET precursor pentagalloylglucose.
The first ET in the ET-pathway, i.e. tellimagrandin II originates from the dehydrogenation of two spatially adjacent galloyl groups of PGG, thus forming the 3,4,5,3´,4´,5´-HHDP residue. The subsequent biosynthetic steps from tellimagrandin II onwards have not been proved, but the proposed biosynthetic steps are largely accepted. For example, it is generally assumed that casuarictin is a product of further dehydrogenation of two spatially adjacent galloyl groups of tellimagrandin II. Similarly, the anomeric centre of ETs can be degalloylated to produce e.g. tellimagrandin I, of which dehydrogenation produces further pedunculagin.
The biosynthetic connections of various monomeric ET groups and examples of structures are shown below. Oligomeric ETs are formed via oxidative couplings between two galloyl units, or galloyl and HHDP units of two monomers or oligomers. At the moment only one step of ET oligomerization has been verified by enzyme studies, but it is reasonable to assume that all the oligomers are formed from their monomeric constituents via oxidative reactions that are under enzymatic control.
