Tannin oxidation is one of the possible routes how tannin activity is triggered especially against insect herbivores
All tannin chemists know that tannins tend to oxidize relatively easily and this is typically indirectly witnessed by the brown colour present in concentrated tannin extracts. It is also clear that tannin oxidation may be enhanced by the addition of metal ions or by changing the pH of the tannin solution above the pKa value of phenolic hydroxyl groups, i.e. pH >9. For these reasons it was long hypothesized that tannins might derive their anti-herbivore activities by being oxidatively activated, since basic conditions (pH 10 and even higher) in guts of insect herbivores should favour tannin oxidation.
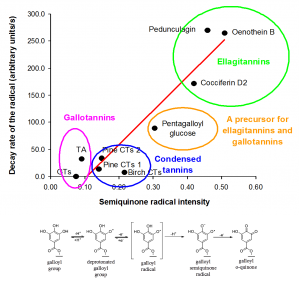
First structure-activity results of tannin oxidation were revealed in 2006 when different types of purified tannins were examined for their ease of oxidation by electron paramagnetic resonance spectroscopy and well-plate based browning assay. Results of these oxidation tests clearly showed that there are clear differences in the ease of oxidation of tannins and that ellagitannins oxidize more rapidly than galloylglucoses, gallotannins and proanthocyanidins.
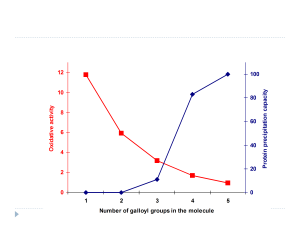
These first results gave insights into the later verified fact that tannins’ pro-oxidant activity is quite clearly inversely related to tannins’ protein precipitation capacity.
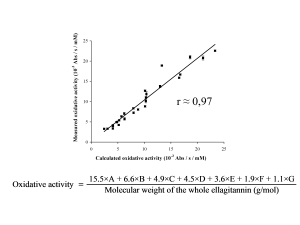
Later we were able to show which types of ellagitannin structures show high or low pro-oxidant activity and these results enabled the formulation of an equation for the calculation of the activity on the basis of the known ellagitannin structure.