Retention times of hydrolysable tannins and flavonoid glycosides tell surprisingly much of their structures as well
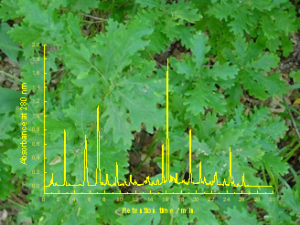 Although retention times of poylphenols as such tell very little or nothing of their chemical structures, the combination of UV and MS spectra with LC retention times can bring about the missing component in compound characterization. Of course all this requires prior knowledge of how modifications in e.g. tannin structures affect their retention behavior. Much of this is typically achieved by the help of purified compounds. At its best retention data in LC analysis or gel chromatographic purification may even reveal magnitude of the bioactivities that are connected with the studied structures.
Although retention times of poylphenols as such tell very little or nothing of their chemical structures, the combination of UV and MS spectra with LC retention times can bring about the missing component in compound characterization. Of course all this requires prior knowledge of how modifications in e.g. tannin structures affect their retention behavior. Much of this is typically achieved by the help of purified compounds. At its best retention data in LC analysis or gel chromatographic purification may even reveal magnitude of the bioactivities that are connected with the studied structures.
For instance, retention times of hydrolysable tannins combined with their UV spectral information reveal much of the galloyl and HHDP load of the tannins and may even clarify if the detected LC peaks belong to C-glycosidic or glucopyranose-based ellagitannins. All this data enables the estimation whether the species containing those specific hydrolysable tannins may show relatively high pro-oxidant activity or alternatively protein precipitation capacity.
For examples how retention times of hydrolysable tannins can be used to estimate their structures and bioactivities, see the following papers:
