Tannins have a number of functional properties in addition to their protein affinity and you should be able to choose the correct property to focus on, and it could guide you to find the most relevant defense compounds in your study plant
Functional properties of plant samples may be linked to their bioactivity
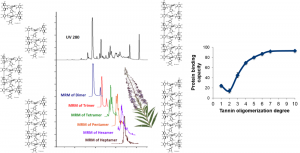
Figure 1. If you know that protein binding capacity is the main functional property determining Epilobium angustifolium bioactivity, you can either measure all individual compounds responsible for this property by UHPLC-MS/MS (top) or simply measure this functional property as such by isothermal titration calorimetry (bottom). Which approach is easier depends on the equipment available. Of course the easiest approach would be to replace isothermal titration calorimetry by e.g. rapid well-plate reader methods designed for protein affinity studies.
Although it is often wise in the field of chemical ecology to focus on the analysis of individual compounds or groups of compounds, sometimes the analysis of functional properties of plant samples can be at least as wise, or even wiser. All depends on the questions asked and of the purposes of the conducted research. If one knows for sure the main property of a plant species that is responsible for its biological activity, then it may make a lot of sense to focus on the measurement of that functional property as carefully as possible (see Fig. 1).
What is highly beneficial in these kinds of measurements of functional properties is that the statistical analyses get much simpler with just one or two parameters linked to each sample in the final data. With one measured parameter 50 samples may be enough to obtain statistically significant results. But if you measure 50 individual compounds responsible for the functional property, then those same 50 samples certainly are not enough to do proper compound-specific statistics. Then you must increase sample numbers considerably, if you want to focus on tens of individual compounds. This certainly is one additional factor explaining why the measurement of functional properties tends to be more popular than the analyses of individual compounds. This is especially true with such functional properties that are easy to measure. Unfortunately, sometimes the easiest methods are not the most relevant ones biologically or chemically. We should be thus able to offer easier ways to measure individual compounds, if we wanted to promote such analyses.
The protein precipitation capacity still has its place in chemical ecology
The functional property most often linked to tannins is their protein precipitation capacity. In studies of chemical ecology it may be quite relevant if it is linked to e.g. ruminants, primates or other types of mammals with acidic gut region. If it is tried to be heavily linked to plant-insect interactions, then its use may be less relevant, given that insect gut conditions may be able to inhibit tannin-protein interactions. As we know today, gut surfactants can make this functional property non-functional and the same can be achieved with alkaline gut conditions. These are found especially with lepidopteran larvae making protein precipitation capacity less important functional property against these types of herbivores.
However, we cannot rule out the possibility that if and when some portion of tannins and plant proteins get into contact before the alkaline gut, then tannins’ protein affinity might play a role in affecting protein availability, even in insects. Naturally all insects do not have alkaline guts making their case again perhaps relevant from the protein affinity point of view. It is likely that we do not know enough at the moment of the varying gut conditions of different insect lineages so that we would be able to make broad conclusions of the big picture. Thus more work is needed.
It is interesting how this same property may be one of the most significant ones when we think of how tannins could protect proteins from being degraded in the rumen of ruminants, to be later released in the small intestine. All of a sudden a harmful property turns into a desired one. It is all about the organism we are studying and in these cases pH plays a pivotal role. In ruminants the pH changes during the digestive tract and this makes the whole aspect of tannin-protein interactions both intriguing and challenging. There simply is no way to simplify this interaction and to have just one truth that would be able to explain all phenomena linked to tannins and proteins. We need more work on the details, before the big picture is clear.
Tannin and polyphenol oxidation – detoxification or not?
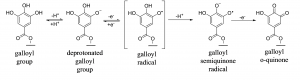
Figure 2. An example of the oxidation pathway for polyphenols. Galloyl groups present in hydrolysable tannins may undergo one-electron oxidation yielding galloyl semiquinone radicals that can be detected by EPR spectroscopy, and further to o-quinones that can be detected as yellow products with spectrophotometry at 415 nm.
It is surprising that at some point it was thought that the alkaline midgut of insects functions as a detoxification mechanism against tannins and other polyphenols, since they tend to be oxidized in these conditions. In a way this makes sense, since the original tannin structures are lost due to oxidation and thus these tannins are no longer able to bind non-covalently to proteins. This in turn would make proteins better available for the herbivore. But if you think what happens to the tannins once oxidized, then it does not look like a detoxification mechanism at all.
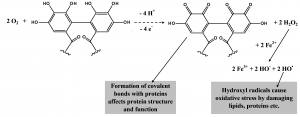
Figure 3. The oxidation of the HHDP groups present in ellagitannins in the presence of molecular oxygen may yield both o-quinones and hydrogen peroxide. That in turn may oxidize iron ions and thus produce hydroxyl radical. The o-quinones may bind covalently with proteins and hydroxyl radicals may cause oxidative stress to other gut components.
We now know that tannin oxidation may yield oxidation products such as quinones that can bind with proteins, but this time covalently (Figs 2-3). Other oxidation productions such as hydrogen radicals and superoxide anion radicals may further oxidize proteins and other gut contents. These processes may thus alter protein structures and cause oxidative stress to herbivores. Both oxidative stress and protein oxidation has been verified to take place in vivo in the midgut of lepidopteran larvae.
A lot is known of the oxidative activity of individual compounds
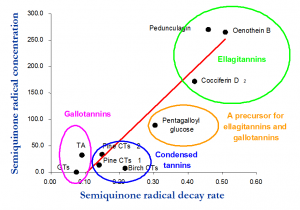
Figure 4. The production of semiquinone radicals and their decay rates into quinones for three ellagitannins (green circle), one galloylglucose (yellow), three mixtures of proanthocyanidins (blue) and two mixtures of gallotannins (purple). This analysis showed that ellagitannins were the most oxidatively active tannins, while both proanthocyanidins and gallotannins were much less effective. For details, see Barbehenn et al. 2006.
The importance of the oxidative activity of polyphenols is not yet clear in the big picture. This gab in knowledge relates to the low number of detailed studies conducted around this subject. Practically all of the studies with purified model tannins are done by the Natural Chemistry Research Group, or together with Dr. Raymond Barbehenn’s group. These studies have clearly shown how ellagitannins as a group are the most active tannins (Fig. 4), but they also differ more than 7-fold in their activities (Fig. 5). Proanthocyanidins have been the least active tannins, especially the procyanidin-rich structures, while prodelphinidin-rich structures have been much more sensitive to oxidation.
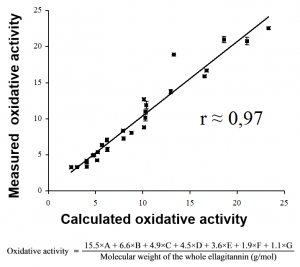
Figure 5. Moilanen & Salminen (2008) showed how 27 purified ellagitannins differed 7-fold in their oxidative activities. They used the structure-activity patterns to create an equation for the calculation of the activity directly from the chemical structure. For details, see Moilanen & Salminen (2008).
We have now at least two tools available for studying the oxidative activity of pure tannins. The first one, i.e. electron paramagnetic resonance spectroscopy methods, was published in 2006. It can be used to measure the formation and decay rates of semiquinone radicals that are formed upon tannin oxidation (Figs 2 and 4). When this method was first used for different types of tannins, it was found out that the components of tannic acid, i.e. gallotannins, are really the least likely tannins to get oxidized at high pH.
These studies were continued by the well-plate reader methods than enabled the measurement of the end products of the phenolic oxidation pathway, the o-quinones. These experiments enabled us to reveal very clear structure-activity patterns within the ellagitannins. This way we constructed the mathematical equation (Fig. 5) to calculate the ellagitannin oxidative activity by simply looking at its chemical structure.
Do not try to reveal irrelevant bioactivities – think about the pH, at least
We at the Natural Chemistry Research Group have worked on this subject since 2002 and in 2011 we launched a simplified method for measuring the overall oxidative activity of any plant sample (Fig. 6). This method operates at pH 10 and it is only relevant, if the pH of the study organism is the same, pH 10. It makes no sense to use this method to verify the significance of polyphenol oxidative activity in plants against certain herbivores, if e.g. the midgut pH of these herbivores is pH 8. In this case the method needs to be operated at pH 8 instead, which naturally gives much lower oxidative activity results than the method that is operated at pH 10. In other words: you benefit by knowing your study organism before choosing your methods. Do think about the pH.
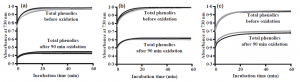
Figure 6. The Salminen & Karonen (2011) assay was developed to enable the measurement of oxidative activities of plant extracts by simply measuring the total phenolic levels of extracts before and after oxidation at high pH. The figure shows total phenolic levels for three plant extracts before and after oxidation. Sample (a) was clearly the most active and sample (c) the least active.
If the midgut pH of an herbivore is e.g. pH 7, or if the plant species studied does not contain any such polyphenols that are easily oxidized, then it is apparent that the oxidative activity as such may not be the most relevant activity type in this specific plant-herbivore interaction. Interestingly, the situation may change dramatically, if polyphenols of this species are easily oxidized with plant’s enzymes such as polyphenol oxidases. Oxidative activity is thus not all about pH. In addition to oxidative enzymes, metal ions may oxidize polyphenols as well.
Our tools can reveal what are the active compounds in each plant species
As noted above, there is no point trying to verify the significance of oxidative activation of polyphenols with such plant species that do not contain any polyphenols that get easily oxidized. With this kind of species the oxidative activity results will be very low and it is very likely that such low levels will not reveal any correlations with insect performance.
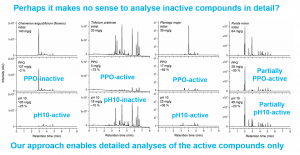
Figure 7. Vihakas et al. (2014) launched a method that uses both the Salminen & Karonen (2011) assay for polyphenol oxidation, but also analyses the oxidation products by UPLC-DAD-MS. This way all the active and inactive polyphenols can be specifically pinpointed to help the future studies to focus on the active species and active compounds only.
We have introduced a method that can be used to screen tens and hundreds of species for their overall oxidative activity. At the same time this method can reveal the individual oxidatively active polyphenols. This is a useful way of finding out e.g. which of the 50 polyphenols found in a plant tissue could contribute to its oxidative activity. If for instance seven active compounds are found and quantified individually, then the 43 inactive compounds could be ignored in the quantification. This way we could simplify a lot our quantitative efforts and we could end up seeing much more specific results in the future. Also the sample numbers could become lower, since instead of 50 compounds, we could focus on seven compounds, making the statistics less demanding from the sample number point of view. Figure 7 below shows an example of such a screening experiment. More information will be available from the section Oxidative activity of tannins.
“Tannin oxidation produces tannin polymers – it is as simple as that. End of story.”
