Sometimes qualitative analysis of polyphenols is quite simple and this is typically the case with the color reactions that are specific to certain types of polyphenols only
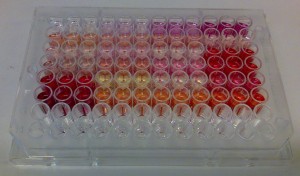 In the absence of sophisticated methods – or even with them – researchers often rely on spectrophotometric evidence when they look for the presence of e.g. proanthocyanidins. This makes a lot of sense as these polyphenols form colorful anthocyanidins upon acid-catalyzed depolymerization reaction in the HCl/butanol-medium. These cannot be mixed with the natural anthocyanidins present in the original extract, if the extract was measured for the presence of anthocyanidins before the depolymerization reaction. Thus this method is very reliable qualitatively and it is much used as a quantitative tool as well, although it does not perform in this task quite as nicely.
In the absence of sophisticated methods – or even with them – researchers often rely on spectrophotometric evidence when they look for the presence of e.g. proanthocyanidins. This makes a lot of sense as these polyphenols form colorful anthocyanidins upon acid-catalyzed depolymerization reaction in the HCl/butanol-medium. These cannot be mixed with the natural anthocyanidins present in the original extract, if the extract was measured for the presence of anthocyanidins before the depolymerization reaction. Thus this method is very reliable qualitatively and it is much used as a quantitative tool as well, although it does not perform in this task quite as nicely.
It is relatively simple to combine spraying reagents with TLC or paper chromatography. The color formed may be witnessed with or without UV light. For instance, proanthocyanidins form a pink or reddish spot with HCl/vanillin reagent. Galloyl esters may be detected on paper chromatograms by their violet to dark blue absorption in UV light enhanced by fuming with ammonia vapour. Also a saturated solution of potassium iodate may be used, giving a rose pink colour. ETs have a dark blue absorption in UV light and give a distinctive purple red colour with a spray of nitrous acid; with NaNO2-AcOH reagent the colour is mentioned to be reddish brown. Another spraying reagent is FeCl3 that gives a dark blue colour with ETs, although it has been inconsistently used also for the detection of GTs.
For more information of the use of color reactions, e.g. in conjunction with the hydrolysis of hydrolysable tannins, have a look at the section Quantitative Analysis of Plants.
